Energy conservation in the Ech-containing thermophilic acetogenic bacterium Thermoanaerobacter kivui (DFG BA5757/2-1)
We are studying the redox and energy metabolism of the thermophilic acetogenic bacterium Thermoanaerobacter kivui, in collaboration with the group of Prof. Dr. Volker Müller (Goethe University Frankfurt / Main). As mesophiles like the model acetogen Acetobacterium woodii, T. kivui grows by converting (4) H2 + (2) CO2 to 1 acetate (Hess et al., 2014). Its redox and energy metabolism, however, differs from that of A. woodii (Fig. 1), especially, since it contains two energy-converting hydrogenases as sole membrane-bound complexes that build up an electrochemical membrane potential. Using genetic tools, biochemistry and classical microbiological experiments, we are currently elucidating the function of both Ech’s during growth on H2+CO2, CO (Schwarz et al., 2020), but also during growth on “heterotrophic” substrates such as sugars. Beyond the role of the Ech’s, we are currently studying the involvement of other important proteins for the redox and energy metabolism of T. kivui (Moon et al., 2019; Jain et al., 2020).
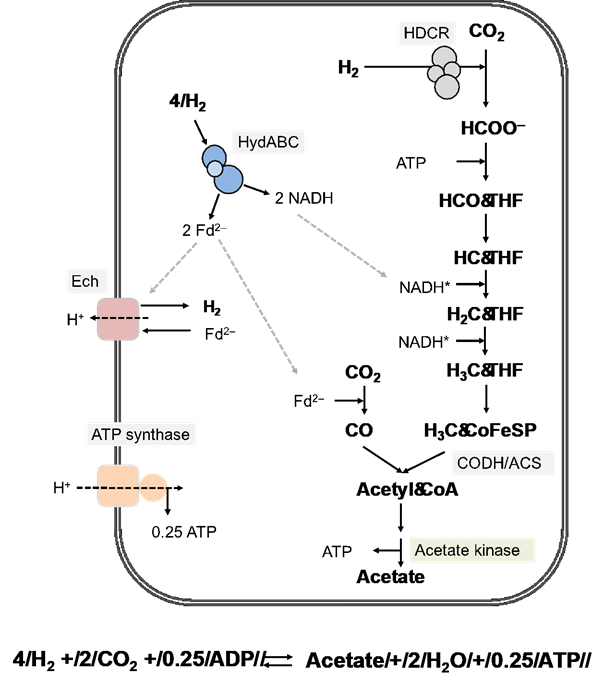
Figure 2. Model of acetogenesis from H2 + CO2 in Thermoanaerobacter kivui (modified after Basen and Müller 2017; Hess et al. 2014). CO2 reduction proceeds via the Wood-Ljungdahl pathway (WLP), yielding acetyl-CoA. In the first step of the methyl branch of the WLP, CO2 reduction to formate is likely catalyzed by a soluble hydrogen-dependent carbon dioxide reductase (HDCR) similar as in A. woodii (Schuchmann and Müller 2013). The electron donors of methylene-THF dehydrogenase and methylene-THF reductase are unknown, therefore, NADH was included in the model (indicated with *). Methylene-THF reductase may be electron-bifurcating. Reductant (NADH and ferredoxin, Fd) is provided by H2 oxidation catalyzed by electron-bifurcating hydrogenase (HydABC). ATP is formed by acetate kinase, but consumed by Formyl-THF ligase (see Fig. 1). Energy is likely conserved by a (Fd)-oxidizing energy converting hydrogenase (Ech).
Team members involved:
Jun.-Prof. Dr. Mirko Basen
Benjamin Zeldes, Ph.D.
MSc Christoph Baum
References:
Hess, V., Poehlein, A., Weghoff, M.C., Daniel, R., and Müller, V. (2014) A genome-guided analysis of energy conservation in the thermophilic, cytochrome-free acetogenic bacterium Thermoanaerobacter kivui. BMC Genomics15: 1139.
Jain, S., Dietrich, H.M., Müller, V., and Basen, M. (2020) Formate Is required for growth of the thermophilic acetogenic bacterium Thermoanaerobacter kivui lacking hydrogendependent carbon dioxide reductase (HDCR). Front Microbiol11: 59.
Moon, J., Henke, L., Merz, N., and Basen, M. (2019) A thermostable mannitol-1-phosphate dehydrogenase is required in mannitol metabolism of the thermophilic acetogenic bacterium Thermoanaerobacter kivui. Environ Microbiol21: 3728-3736
Schwarz, F.M., Ciurus, S., Jain, S., Baum, C., Wiechmann, A., Basen, M., and Müller, V. (2020)
Revealing formate production from carbon monoxide in wild type and mutants of Rnf- and Ech-containing acetogens, Acetobacterium woodii and Thermoanaerobacter kivui. Microb Biotechnol13: 2044-2056.
gefördert durch die

